Introduction
Catalysts are used extensively in many branches of chemistry and the search for better catalysts is never-ending. The principal objective of this work is to acquaint the people with basic concept of catalysis in the specific area of transition metal catalysts. Some examples of chemical reaction on transition metal catalysts are illustrated to explan the role of transition metal. In addition, the principal of orbital symmetry conservation and its application in transition metal catalyzed transformations was illustrated.
1. What is a catalyst?
1.1 Background
Catalysts are very widely used industrially. Most industrially important chemical reactions involves catalysis. In addition, most biochemically significant processes are catalysed. Research into catalysis is a major field in applied science and involves many areas of chemistry, notably in organometallic chemistry and materials science. Catalytic reactions are preferred in environmentally friendly green chemistry due to the reduced amount of waste generated,as opposed to stoichiometric reactions in which all reactants are consumed and more side products are formed. Catalysts could speed up the reaction that you want. This minimizes the amount of unwanted biproducts and produce the desired material rapidly[1].
Two general types of catalyst are used: homogeneous and heterogeneous. A homogeneous catalyst exists in the same phase as the reactants and products. So if a reaction was carried out in solution, the catalytically active species would also be in solution. A heterogeneous catalyst exists in a different phase from the reactants and products. Typically, the catalysts is a solid and the reactants and products are liquids/gases. The reaction takes place at the solid surface[2].
1.2 General Principal
1.2.1 Reaction energy
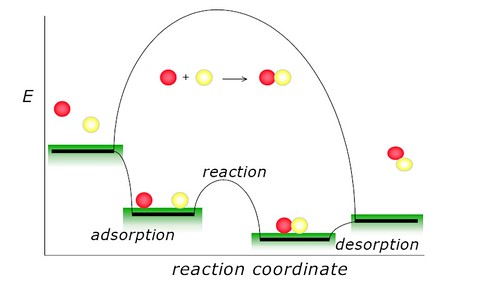
Catalysts work by changing the activation energy for a reaction, i.e., the minimum energy needed for the reaction to occur. This is accomplished by providing a new mechanism or reaction path through which the reaction can proceed. When the new reaction path has a lower activation energy, the reaction rate is increased and the reaction is said to be catalyzed[3]. This figure could show clear what a catalyst contribute in the reaction. Catalyst is not related to the thermodynamics of the process[4].
A catalyst is a substance that increases the rate of a reaction without being consumed in the reaction, so catalyst can make the chemical reaction happen at a lower energy cost.
1.2.2 Catalysis process
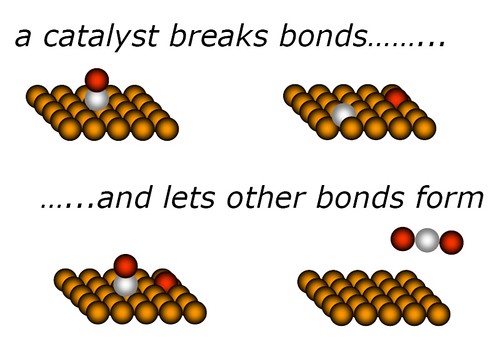
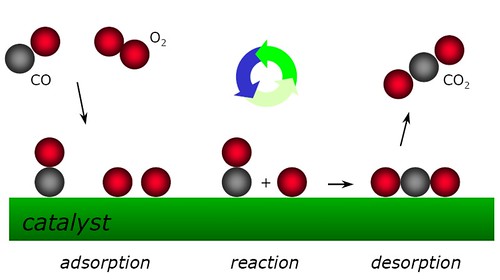
All technologically important chemical reactions involve the breaking or formation of a chemical bond[5].Taking the CO oxidation as an example. The catalysis process can be illustrated as the following figure[4]. catalysis will be favoured if the reactant molecules bind more strongly to the catalyst surface than the product molecules. Ideally, the reactant molecules are concentrated on the catalyst surface, where they undergo far more frequent collisions than if they were in the gas phase. It is suggested that when a reactant is bound to the catalyst surface, the covalent bonds holding its atoms together are distorted and weakened, thus making them more susceptible to breaking.Actually, the mechanistic origin of catalysis is complex.Generally speaking, catalyst intervene by changing the reaction mechanism, providing pathways that require lower energies of activation, thus increasing the reaction rates[6].
Kinetically, catalytic reactions behave like typical chemical reactions, i.e. the reaction rate depends on the frequency of contact of the reactants in the rate-determining step. Usually, the catalyst participates in this slow step, and rates are limited by amount of catalyst. In heterogeneous catalysis, the diffusion of reagents to the surface and diffusion of products from the surface can be rate determing[1].
2. Why transition metal ?
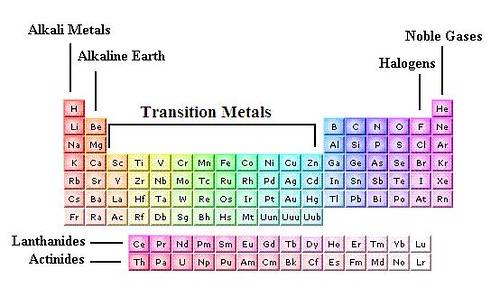
2.1 what is transition metal ?
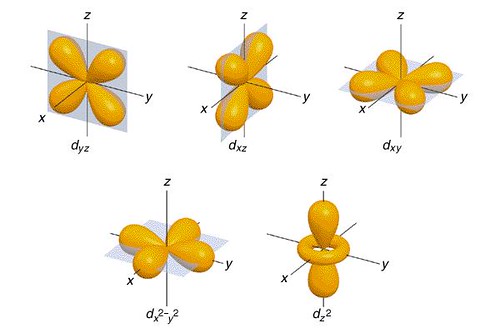
The d orbitals are what give transition metals their distinguished properties. The transition metal ions the outermost d orbitals are incompletely filled with electrons so they can easily give and take electrons. This makes transition metals prime candidates for catalysis. The outermost s and p orbitals are usually empty and therfore less useful for electron transfer[2]. Below are representations of the d orbitals[7].
2.2 The excellent properties of transition metal
The principal reasons why transition metals contribute the essential ingredient in catalyst systems can be summarized as the following headings[2]:
(a) Bonding ability
(b) Catholic choice of ligands
(c) Ligand effects
(d) Variability of oxidation state
(e) Variablility of co-ordination number
A d-block metal ion has nine valence shell orbitals to accommodate its valence electrons and to form hybrid molecular orbitals in bonding with other groups. The d orbitals are illustrated in the following figure [8]. The special configuration enable the d metal to form both σ- and π- bonds which is one of the key factors in imparting catalytic properties to the transition metals and their complexes.
2.3 Transition metal complexes
There are two commonly used approaches to describing the bonding in transition metal coordination complexes : “Crystal Field Theory” and molecular orbital theory. Crystal Field Theory is an electrostatic approach where we treat the ligands as point negative charges and ask ourselves what the effect of repulsion between these charges and the d-electrons on the metal ions will be. Molecular orbital theory is more complex than crystal field theory, but it allows a more complete explanation of the observed physical properties.
Transition metal complexes are important in catalysis. When compounds of these metals form stable complexes with Lewis base ligands, the coordinate sharing of electron pairs on the ligand molecules may produce a coordinatively saturated (18-electron) or an unsaturated (16, 14 or fewer electron) valence shell configuration. Transition metal compounds would have limited interest and importance if they did not undergo chemical reactions[9].
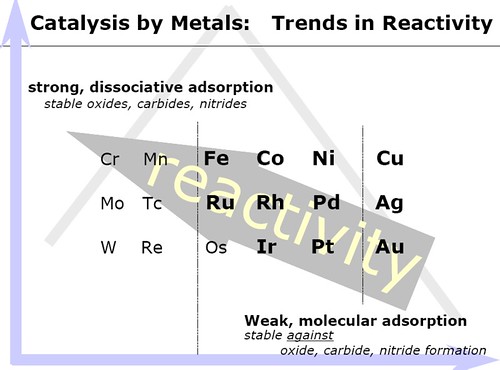
2.4 Sabatier principle
The Sabatier principle is named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the substrate should be "just right"; that is, neither too strong nor too weak. If the interaction is too weak, the substrate will fail to bind to the catalyst and no reaction will take place. On the other hand, if the interaction is too strong, the catalyst gets blocked by substrate or product that fails to dissociate[4,10].
The following figure shows a volcano plot for the decomposition of formic acid using different transition metals as catalysts.[4].
2.5 Case study: CO adsorption on transition metal
CO adsorption is an important mechanistic step in the chemical reaction pathway of many heterogeneous catalytic reactions[11]. In these catalytic reactions, transition metals play a central role in enhancing considerably the rate of reaction that involves theadsorption, diffusion and bond dissociation steps of carbon monoxide. Consider the case of carbon monoxide adsorption on transition metal as illustrated in Fig.5 which has been widely investigated [4].
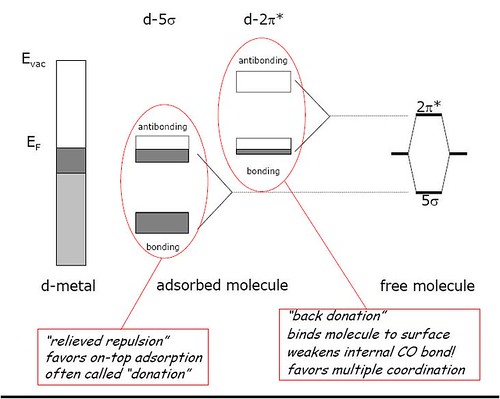
The σ component is formed by interaction between an empty metal σ-orbital and a filled sp carbon orbital. The π-component is formed by interaction between a filled metal d π- or hybrid dp π- orbital, and one of the sets of empty antibonding p π-orbitals of the carbon monoxide. The σ-component results in a net transfer of electron density from the ligand to the metal and π-component in a net transfer in the opposite direction. The bonding is synergistic and results in a decrease in the C-O bond order[2].
From the above example, it should be clear that transition metal catalysts can readily form strong bonds with compounds containing π-electron systems or having orbitals of suitable symmetry/energy to form dπ-bonds.
3. Molecular orbital symmetry conservation in transition metal catalyzed transformations
3.1 Molecular orbital symmetry conservation
Woodward and Hoffmann have published a series of communications extending simple molecular orbital theory into the area of reaction chemistry[12]. The stereochemistry of pericyclic reactions based on orbital symmetry could be predicted by this rule. These include electrocyclic reactions, cycloadditions, and sigmatropic reactions. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model, while Woodward had died two years before he could win a second Nobel Prize for Chemistry[13].
According to this rule, a reaction is allowed only when all the reactant bonding electrons are transferred without a symmetry-imposed barrier, into bonding orbitals of the products. Explains the relationship among the structure and configuration of the reactant, the conditions (thermal or photochemical) under which the reaction takes place, and the configuration of the products[14].
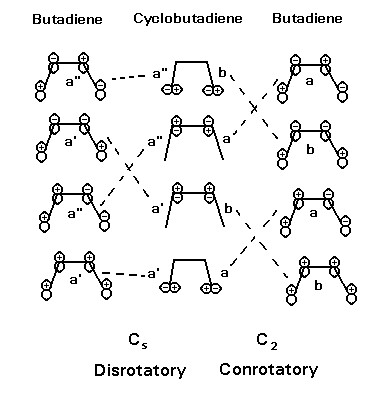
Consider the butadiene ->cyclobutene reaction. If we consider the ring opening of cyclobutene, there are two pathways, conrotatory or disrotatory. The diagram shows these paths by considering two possible rotations of the CH2 groups.
Conrotatory motion maintains C2 symmetry, and disrotatory motion maintains Cs symmetry. We may therefore draw an orbital energy correlation diagram, in which the orbitals of butadiene are classified under Cs symmetry, and under C2 symmetry . The bonds of cyclobutene involved are shown in the middle. Lines are then drawn connecting the cyclobutene orbitals under Cs symmetry and under C2 symmetry.The diagram clearly shows that the disrotatory path is favoured photochemically and the conrotatory path is favoured thermally[14].
3.2 Application in transition metal
The Woodward-Hoffmann postulate, dividing molecular transformations into “allowed” and “forbidden” categories, has proven a powerful tool for understanding a large body of complex chemistry. This communication extends the concept of symmetry conservation into transition metal catalysis.
The concerted fusion of two olefins to a ground-state cyclobutane ring requires the introduction of two electrons
into the olefin AS orbital and the removal of two electrons from the olefin SA orbital (A = antisymmetric;
S = symmetric).A transition metal system containing d orbitals of sufficient energy and possessing the appropriate number of d electrons can carry out these operations.
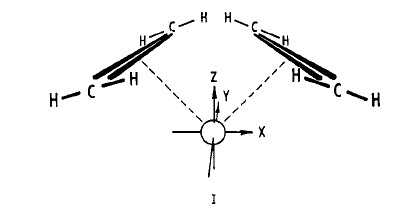
Consider the metal-diolefin complex I.
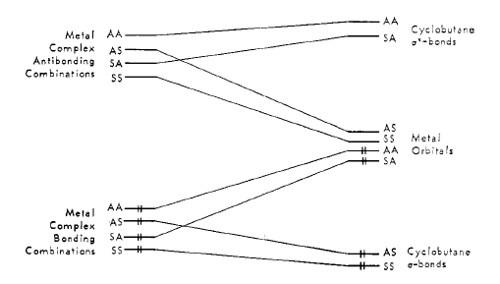
Metal orbitals of the appropriate energy could combine with the olefin orbitals yielding a set of new molecular orbitals of the same symmetry classification; bonding between the metal and the olefin, then, could result with electronic population of the bonding molecular orbitals.A correlation diagram for the cyclobutanation of the ligand-bound olefins is illustrated in the following figure, which describes an orbital pathway for the placement of electron pairs into the cyclobutane σ bonds and for the removal of the appropriate olefin π electrons[15].
The application of symmetry conservation concepts to the selected reactions introduces a novel mechanism involving a role for the transition metal unique in catalysis. It suggest that certain metal systems containing orbital configurations of the prerequisite energy are capable of rendering otherwise forbidden cycloaddition reactions allowed by providing a template of atomic orbitals through which electron pairs of transforming hydrocarbon ligands and metal systems can interchange and flow into the required regions of space.
Reference
[1] http://en.wikipedia.org/wiki/Catalysis
[2]Christopher Masters, Homogeneous TransitionMetal Catalysis A Gentle Art, Chapman and Hall, New York. 1981.
[3]http://www.infoplease.com/ce6/sci/A0857209.html
[4]http://www.catalysis.nl/kica/files/What_is_Catalysis.pdf
[5] Elizabeth Santos, Wolfgang Schmickler, "d-Band Catalysis in Electrochemistry", ChemPhysChem 2006, 7, 2282-2285.
[6] http://www.physchem.co.za/OB12-che/catalysis.htm
[7] http://www.chemeddl.org/collections/TSTS/Stahl/Stahlpg5-8/PeriodicTable5to8.html
[8]http://cnx.org/content/m15057/latest/
[9]http://web.chemistry.gatech.edu/~wilkinson/Class_notes/spring_2004_1311_page/slides/Transition%20metal%20coordination%20chemistry%201%20up.pdf
[10] http://en.wikipedia.org/wiki/Sabatier_principle
[11]Constantinos D. Zeinalipour-Yazdi, Andrew L. Cooksy, Angelos M. Efstathiou, "CO adsorption on transition metal clusters: Trends from density functional theory", Surface Science, 2008, 602, 1858–1862.
[12] Roald Hoffmann, Robert B. Woodward, "Conservation of orbital symmetry", Acc. Chem. Res., 1968, 1 (1), 17-22.
[13] http://en.wikipedia.org/wiki/Woodward-Hoffmann_rules
[14]http://www-theor.ch.cam.ac.uk/people/nch/lectures/T0new/node18.html
[15]Frank D. Mango, Jerry H. Schachtschneider, "Molecular orbital symmetry conservation in transition metal catalyzed transformations", J. Am. Chem. Soc., 1967, 89(10), 2484-2486.